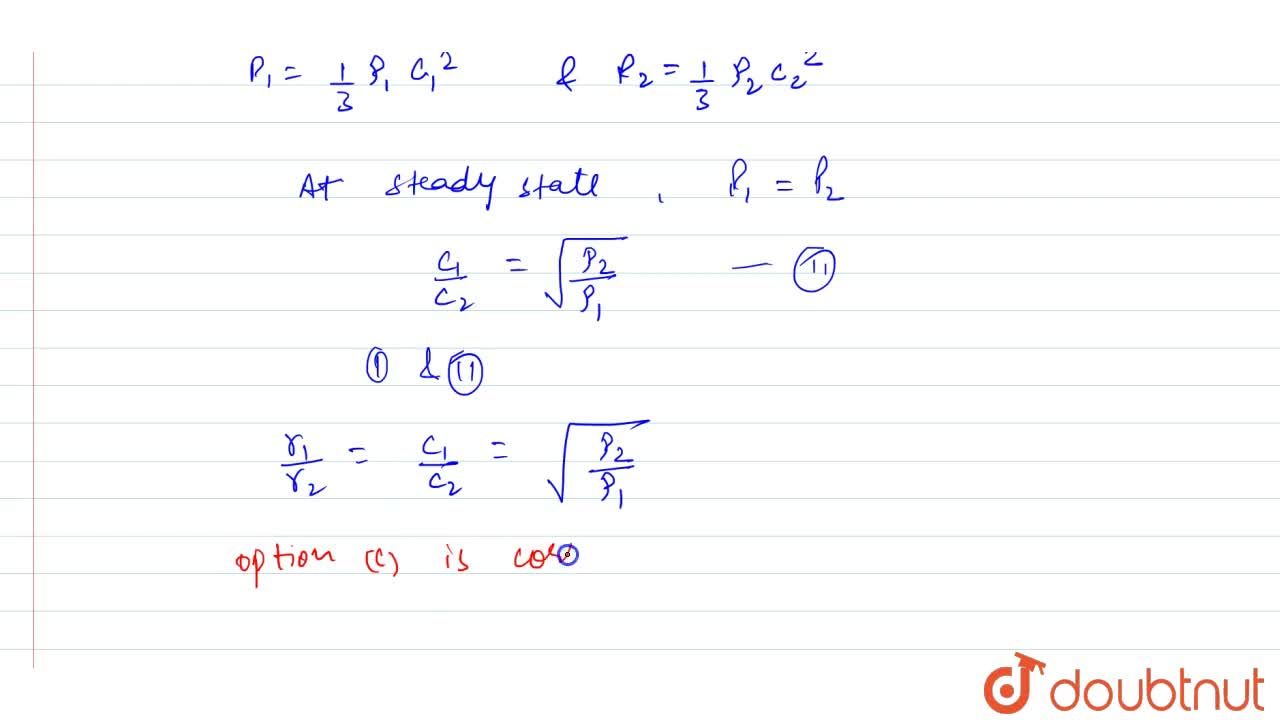
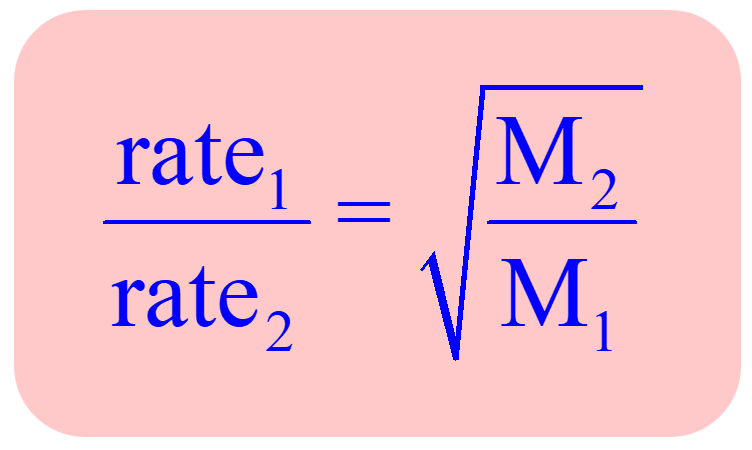50 ml of a gas A diffuse through a membrane in the same time as for the diffusion of 40 ml of a gas B under identical pressure - temperature conditions. If
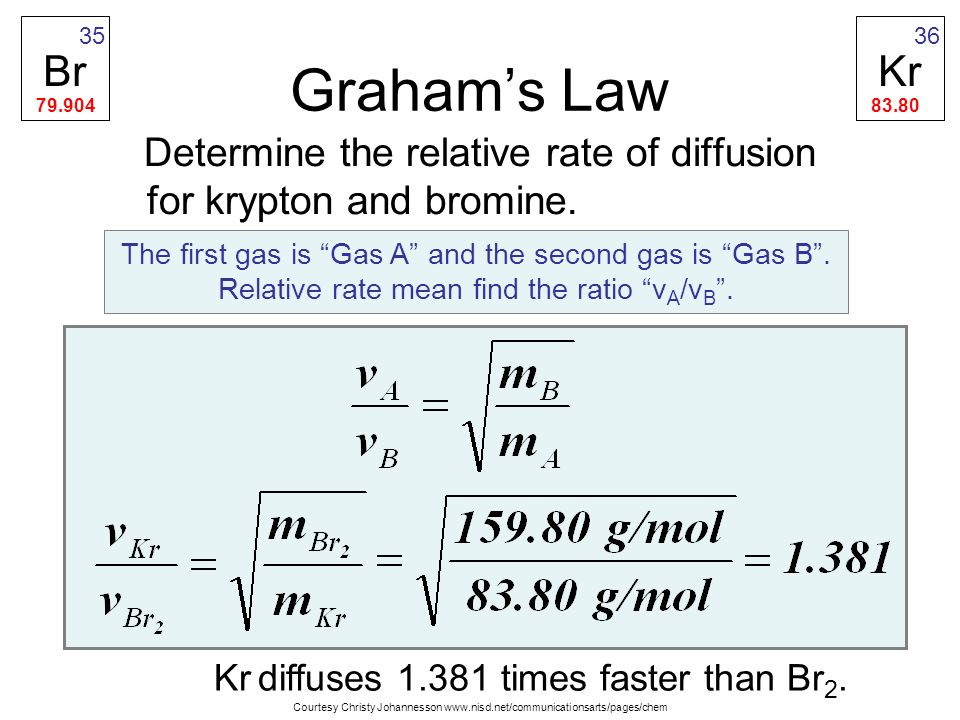
Diffusion vs. Effusion Diffusion - The tendency of the molecules of a given substance to move from regions of higher concentration to regions of lower. - ppt download
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora
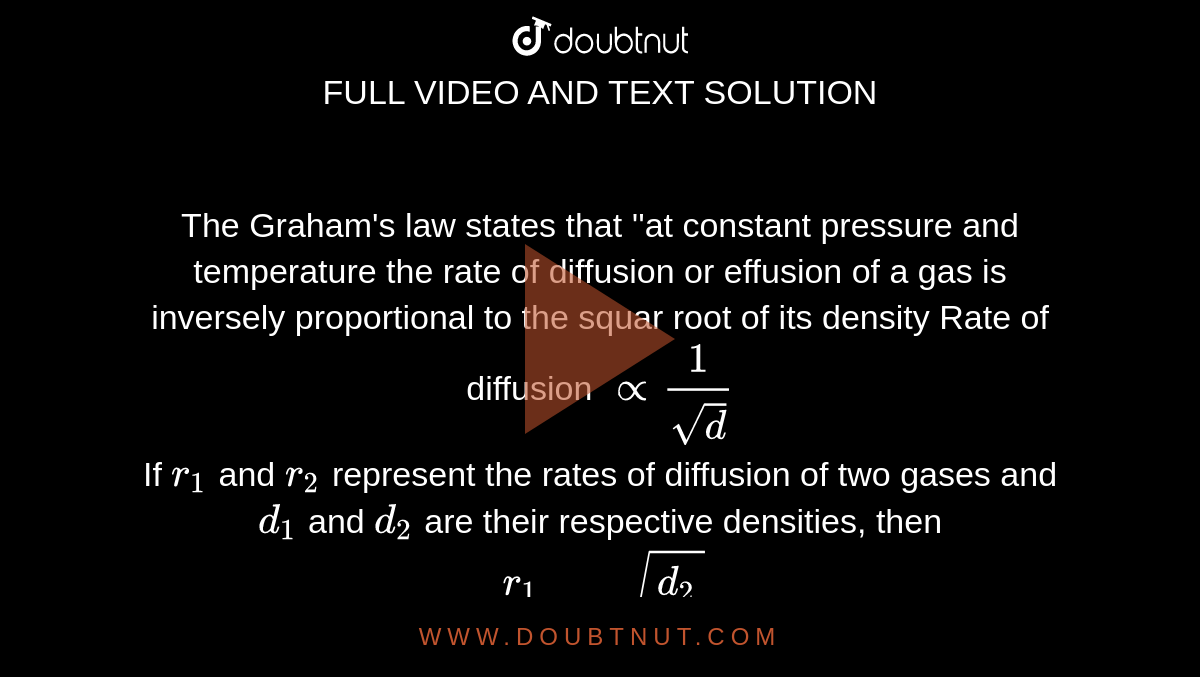
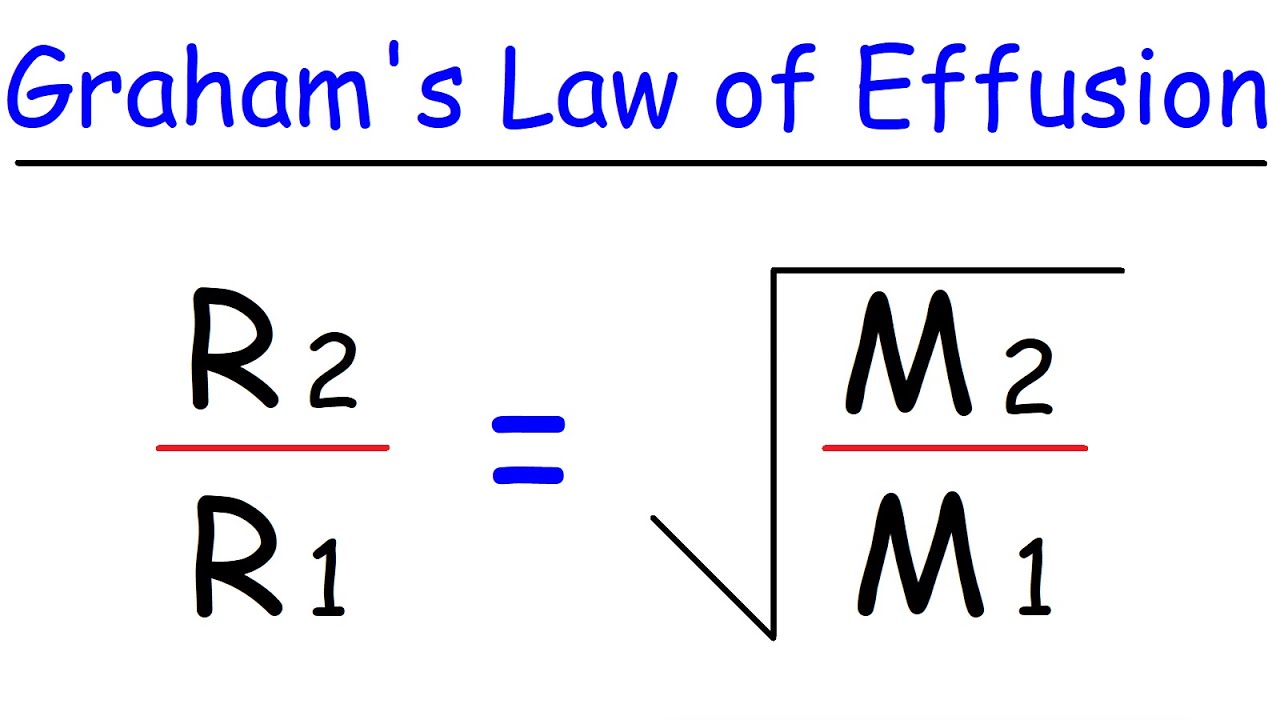
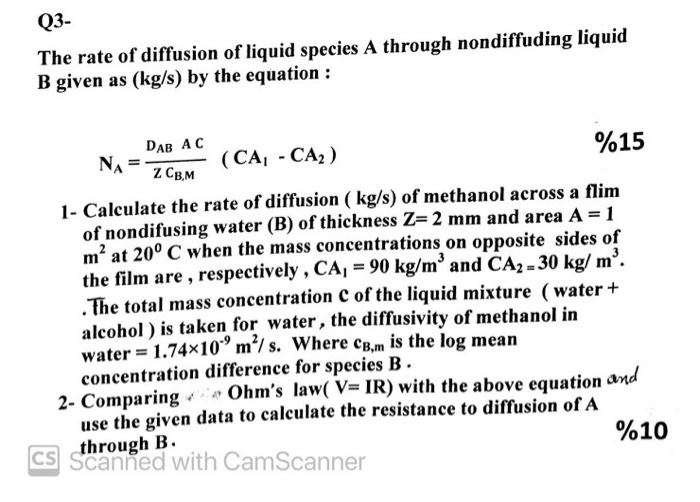
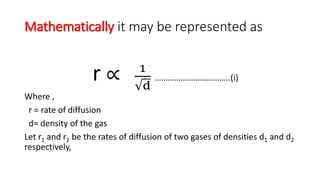
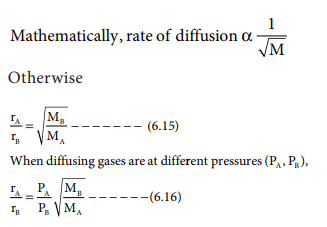
The Graham's law states that ''at constant pressure and temperature the rate of diffusion or effusion of a gas is inversely proportional to the squar root of its density Rate of diffusion